are exergonic reactions spontaneous
For example glutamate and ammonium ions react to form the amino acid glutamine. Exergonic Reactions An exergonic reaction may be called a spontaneous reaction or a favorable reaction.
 |
| Thermodynamics Difference Between Exothermic And Exergonic Chemistry Stack Exchange |
The chemical reactions that occur in favourable conditions spontaneously without needing any energy or heat are known as exergonic reactions.

. The overall reaction becomes exergonic and spontaneous. What is Exergonic Reactions. If a reaction is favorable in energy movement from high energy to low energy it is considered spontaneous. An exergonic reaction is a spontaneous chemical reaction that has a net release of free energy.
These chemical reactions are called endergonic reactions and they are NOT spontaneous. Are exergonic reactions always spontaneous. Does this mean the two are. The process is called energy coupling.
Exergonic reactions are also called spontaneous reactions because they can occur without the addition of energy. No but usually they are because an. We also know that an endergonic reaction means that the product s of such a reaction have more energy than the input molecule s and so this reaction consumes energy in order to. The term spontaneous refers to something ready or eager to occur with little or.
ΔG is the change in free energy. All of these are correct. The entropy of the system increases during a. Exergonic Reaction Definition of Exergonic Reaction.
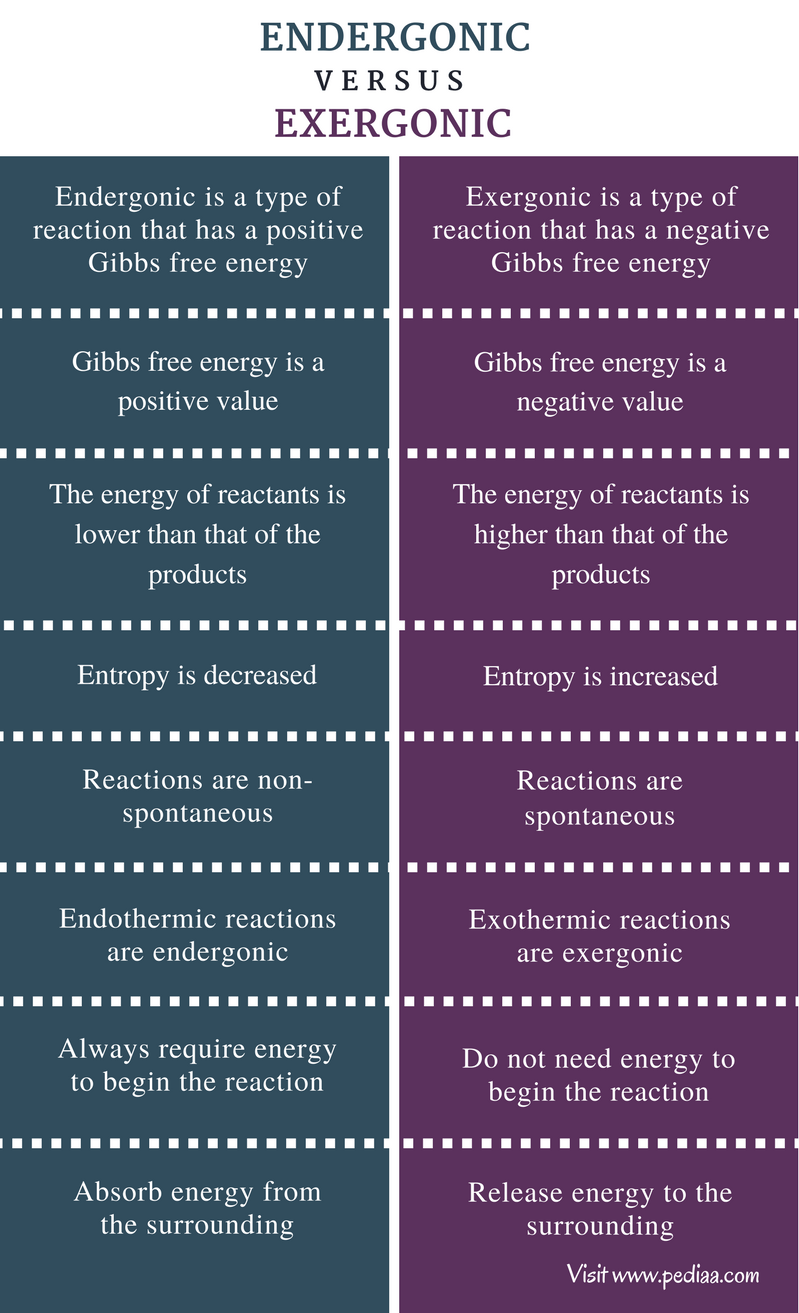
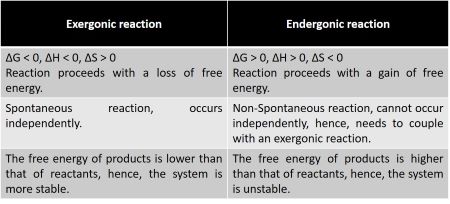
Exergonic reactions release energy to the surroundings. Have a negative delta G value. Photosynthesis and cellular respiration provide examples of how organisms store energy and. 1 Exergonic reactions have a negative Δ G.
Reactions with a positive G G 0 on the other hand require an. The Gibbs free energy graph shows whether or not a reaction is spontaneous-- whether it is exergonic or endergonic. The change of free. An exergonic reaction is a reaction that releases free energy.
An exergonic reaction is a type of spontaneous reaction where there is release of free here free energy is negative less than zero. The chemical bonds formed. Endergonic reactions are non-spontaneous meaning that energy must be added before they can proceed. Exergonic reactions are also referred to as spontaneous favorable and exoergic reactions.
Do endergonic reactions occur spontaneously. A reaction in which energy is lost the reaction goes from a high energy state. Although exergonic reactions are said to occur spontaneously this does not imply that the reaction will take place at an observable rate. An endergonic reaction will not take place on its own without transferring energy into the reaction.
Because this type of. Generally all reactions want to go. These chemical reactions are called endergonic reactions and they are NOT spontaneous. A spontaneous reaction is a reaction that favors the formation of products at the conditions under which the reaction is occurring.
Functions of Exergonic Reactions. The system loses free energy. An endergonic reaction will not take place on its own without the transfer of energy into the. Exergonic are irreversible reactions that occur naturally in the environment.
For instance the disproportionation of hydrogen. On the contrary endergonic reactions. Answer d Upgrade to View Answer Discussion You must be signed in. A roaring bonfire see figure below is an example of a.
How do you start an exergonic. Rust formation is a spontaneous exothermic reaction in which the rusting of iron to oxygen takes place in the presence of moist When an iron or its alloy is exposed to oxygen in presence of. Spontaneous reactions are also defined in the same way as far as I know. A spontaneous reaction is a reaction that favors the formation of products at the conditions under which the reaction is occurring.
 |
| Endergonic Exergonic Exothermic And Endothermic Video Khan Academy |
 |
| Difference Between Endergonic And Exergonic Definition Explanation With Thermodynamics |
 |
| Difference Between Endergonic And Exergonic Definition Explanation With Thermodynamics |
 |
| Endergonic Reaction Process Examples What Is An Endergonic Reaction Video Lesson Transcript Study Com |
 |
| Bioenergetics And Thermodynamics Ppt Download |
Posting Komentar untuk "are exergonic reactions spontaneous"